GLPG-1690(ziritaxestat)
GLPG1690 is a selective autotaxin inhibitor discovered by Galapagos, with potential application in idiopathic pulmonary disease (IPF). In a Phase 1 study in healthy human volunteers, GLPG1690 demonstrated favorable safety and tolerability, as well as a strong pharmacodynamic signal implying target engagement.
For research use only. We do not sell to patients.
Chemical Information
| Name | GLPG-1690(ziritaxestat) |
|---|---|
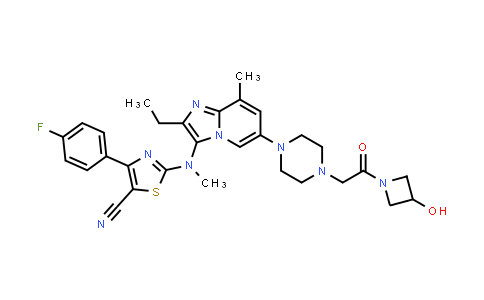
| Iupac Chemical Name | 2-((2-ethyl-6-(4-(2-(3-hydroxyazetidin-1-yl)-2-oxoethyl)piperazin-1-yl)-8-methylimidazo[1,2-a]pyridin-3-yl)(methyl)amino)-4-(4-fluorophenyl)thiazole-5-carbonitrile |
| Synonyms | GLPG-1690; GLPG 1690; GLPG1690; ziritaxestat. |
| Molecular Formula | C30H33FN8O2S |
| Molecular Weight | 588.70 |
| Smile | C(C)C=1N=C2N(C=C(C=C2C)N2CCN(CC2)CC(=O)N2CC(C2)O)C1N(C=1SC(=C(N1)C1=CC=C(C=C1)F)C#N)C |
| InChiKey | REQQVBGILUTQNN-UHFFFAOYSA-N |
| InChi | InChI=1S/C30H33FN8O2S/c1-4-24-29(35(3)30-34-27(25(14-32)42-30)20-5-7-21(31)8-6-20)39-15-22(13-19(2)28(39)33-24)37-11-9-36(10-12-37)18-26(41)38-16-23(40)17-38/h5-8,13,15,23,40H,4,9-12,16-18H2,1-3H3 |
| CAS Number | 1628260-79-6 |
| Related CAS |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | crystalline solid |
|---|---|
| Purity | 98% |
| Storage | 3 years -20ºCpowder |
| Solubility | Soluble in DMSO |
| Handling | |
| Shipping Condition | Shipped under ambient temperature as non-hazardous chemical. |
| HS Code |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Desroy N, Housseman C, Bock X, Joncour A, Bienvenu N, Cherel L, Labeguere V, Rondet E, Peixoto C, Grassot JM, Picolet O, Annoot D, Triballeau N, Monjardet A, Wakselman E, Roncoroni V, Le Tallec S, Blanque R, Cottereaux C, Vandervoort N, Christophe T, Mollat P, Lamers M, Auberval M, Hrvacic B, Ralic J, Oste L, van der Aar E, Brys R, Heckmann B. Discovery of 2-[[2-Ethyl-6-[4-[2-(3-hydroxyazetidin-1-yl)-2-oxoethyl]piperazin-1-yl]-8-methyli midazo[1,2-a]pyridin-3-yl]methylamino]-4-(4-fluorophenyl)thiazole-5-carbonitrile (GLPG1690), a First-in-Class Autotaxin Inhibitor Undergoing Clinical Evaluation for the Treatment of Idiopathic Pulmonary Fibrosis. J Med Chem. 2017 May 1. doi: 10.1021/acs.jmedchem.7b00032. [Epub ahead of print] PubMed PMID: 28414242.
Chemical Structure