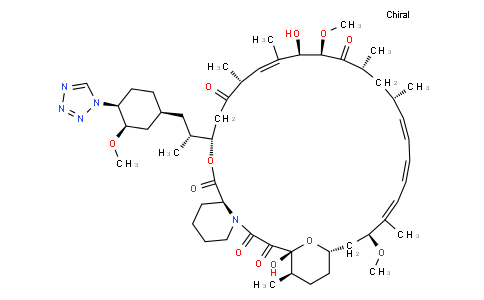
Zotarolimus
Catalog No: 161009018
CAS Number: 221877-54-9
Purity: 98% by HPLC
It is a semi-synthetic derivative of rapamycin. It was designed for use in stents with phosphorylcholine as a carrier. Coronary stents reduce early complications and improve late clinical outcomes in patients needing interventional cardiology. Medtronic are using zotarolimus as the anti-proliferative agent in the polymer coating of their Endeavor and Resolute products.
For research use only. We do not sell to patients.
Chemical Information
| Name | Zotarolimus |
|---|---|
| Iupac Chemical Name | (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-10,21-dimethoxy-3-{(1R)-2-[(1S,3R,4S)-3-methoxy-4-(1H-tetrazol-1-yl)cyclohexyl]-1-methylethyl}-6,8,12,14,20,26-hexamethyl-4,9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-heptadecahydro-3H-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,5,11,28,29(6H,31H)-pentone |
| Synonyms | A-179578; A 179578; A179578; ABT578; ABT-578; ABT 578; Endeavor; Zotarolimus; Rapamycin, 42-deoxy-42-(1H-tetrazol-1-yl)-, (42S)-. |
| Molecular Formula | C52H79N5O12 |
| Molecular Weight | 966.227 |
| Smile | O[C@H]\1[C@H](C([C@@H](C[C@@H](/C=C/C=C/C=C(/[C@H](C[C@@H]2CC[C@H]([C@](C(C(N3[C@H](C(O[C@@H](CC([C@@H](/C=C1\C)C)=O)[C@@H](C[C@H]1C[C@H]([C@H](CC1)N1N=NN=C1)OC)C)=O)CCCC3)=O)=O)(O2)O)C)OC)\C)C)C)=O)OC |
| InChiKey | CGTADGCBEXYWNE-JUKNQOCSSA-N |
| InChi | InChI=1S/C52H79N5O12/c1-31-16-12-11-13-17-32(2)43(65-8)28-39-21-19-37(7)52(64,69-39)49(61)50(62)56-23-15-14-18-41(56)51(63)68-44(34(4)26-38-20-22-40(45(27-38)66-9)57-30-53-54-55-57)29-42(58)33(3)25-36(6)47(60)48(67-10)46(59)35(5)24-31/h11-13,16-17,25,30-31,33-35,37-41,43-45,47-48,60,64H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,32-17+,36-25+/t31-,33-,34-,35-,37-,38+,39+,40+,41+,43+,44+,45-,47-,48+,52-/m1/s1 |
| CAS Number | 221877-54-9 |
| Related CAS |
Ordering Information
| Packaging | Price | Availability | Purity | Shipping Time |
|---|---|---|---|---|
| Bulk | Enquiry | Enquiry | Enquiry |
| Formulation | Solid powder |
|---|---|
| Purity | 98% by HPLC |
| Storage | -20 ºC for 3 years |
| Solubility | Soluble in DMSO |
| Handling | |
| Shipping Condition | Shipped under ambient temperature |
| HS Code |
Coming soon.
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
Not available
Chemical Structure