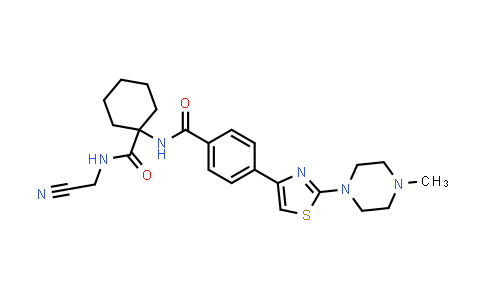
L006235
L006235 is a potent, reversible cathepsin K inhibitor (IC50 = 0.25 nM) that displays > 4000-fold selectivity over cathepsins B, L and S.
仅供研究使用。 我们不向患者出售。
化学信息
| 名称 | L006235 |
|---|---|
| Iupac 化学名称 | N-(1-((cyanomethyl)carbamoyl)cyclohexyl)-4-(2-(4-methylpiperazin-1-yl)thiazol-4-yl)benzamide |
| 同义词 | L006235; L 006235; L-006235. |
| 英文同义词 | L006235; L 006235; L-006235. |
| 分子式 | C24H30N6O2S |
| 分子量 | 466.6 |
| Smile | O=C(NC1(C(NCC#N)=O)CCCCC1)C2=CC=C(C3=CSC(N4CCN(C)CC4)=N3)C=C2 |
| InChiKey | FIVYCSWOCXEWSE-UHFFFAOYSA-N |
| InChi | InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) |
| Cas号 | 294623-49-7 |
| 相关CAS号 |
订购信息
| 包装 | 价格 | 库存 | 纯度 | 备货期 |
|---|---|---|---|---|
| 大货 | 询价 | 询价 | 询价 |
| 外观性状 | 固体粉末 |
|---|---|
| 纯度 | 98% Min. |
| 存储 | 干燥、黑暗,短期(日至周)在0-4摄氏度,长期(月至年)在-20摄氏度。 |
| 可溶性 | 可溶于DMSO |
| 处理方式 | |
| 运输条件 | 作为非危险化学品在环境温度下装运。这种产品在正常运输和海关工作期间可以稳定几周。 |
| 海关编码 |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Soung do Y, Gentile MA, Duong le T, Drissi H. Effects of pharmacological inhibition of cathepsin K on fracture repair in mice. Bone. 2013 Jul;55(1):248-55. doi: 10.1016/j.bone.2013.02.010. Epub 2013 Feb 26. PubMed PMID: 23486186.
2: Hayami T, Zhuo Y, Wesolowski GA, Pickarski M, Duong le T. Inhibition of cathepsin K reduces cartilage degeneration in the anterior cruciate ligament transection rabbit and murine models of osteoarthritis. Bone. 2012 Jun;50(6):1250-9. doi: 10.1016/j.bone.2012.03.025. Epub 2012 Mar 30. PubMed PMID: 22484689.
3: Pennypacker BL, Duong le T, Cusick TE, Masarachia PJ, Gentile MA, Gauthier JY, Black WC, Scott BB, Samadfam R, Smith SY, Kimmel DB. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res. 2011 Feb;26(2):252-62. doi: 10.1002/jbmr.223. PubMed PMID: 20734451.
4: Svelander L, Erlandsson-Harris H, Astner L, Grabowska U, Klareskog L, Lindstrom E, Hewitt E. Inhibition of cathepsin K reduces bone erosion, cartilage degradation and inflammation evoked by collagen-induced arthritis in mice. Eur J Pharmacol. 2009 Jun 24;613(1-3):155-62. doi: 10.1016/j.ejphar.2009.03.074. Epub 2009 Apr 7. PubMed PMID: 19358841.
5: Le Gall C, Bonnelye E, Clézardin P. Cathepsin K inhibitors as treatment of bone metastasis. Curr Opin Support Palliat Care. 2008 Sep;2(3):218-22. doi: 10.1097/SPC.0b013e32830baea9. Review. PubMed PMID: 18685424.
6: Desmarais S, Black WC, Oballa R, Lamontagne S, Riendeau D, Tawa P, Duong le T, Pickarski M, Percival MD. Effect of cathepsin k inhibitor basicity on in vivo off-target activities. Mol Pharmacol. 2008 Jan;73(1):147-56. Epub 2007 Oct 16. PubMed PMID: 17940194.
7: Black WC, Percival MD. The consequences of lysosomotropism on the design of selective cathepsin K inhibitors. Chembiochem. 2006 Oct;7(10):1525-35. Review. PubMed PMID: 16921579.
8: Falgueyret JP, Desmarais S, Oballa R, Black WC, Cromlish W, Khougaz K, Lamontagne S, Massé F, Riendeau D, Toulmond S, Percival MD. Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J Med Chem. 2005 Dec 1;48(24):7535-43. PubMed PMID: 16302795.
9: Palmer JT, Bryant C, Wang DX, Davis DE, Setti EL, Rydzewski RM, Venkatraman S, Tian ZQ, Burrill LC, Mendonca RV, Springman E, McCarter J, Chung T, Cheung H, Janc JW, McGrath M, Somoza JR, Enriquez P, Yu ZW, Strickley RM, Liu L, Venuti MC, Percival MD, Falgueyret JP, Prasit P, Oballa R, Riendeau D, Young RN, Wesolowski G, Rodan SB, Johnson C, Kimmel DB, Rodan G. Design and synthesis of tri-ring P3 benzamide-containing aminonitriles as potent, selective, orally effective inhibitors of cathepsin K. J Med Chem. 2005 Dec 1;48(24):7520-34. PubMed PMID: 16302794.