ML188
ML188 is a Potent Noncovalent Small Molecule Inhibitor of the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) 3CL Protease. The X-ray structure of SARS-CoV 3CLpro bound with ML188 was instrumental in guiding subsequent rounds of chemistry optimization. ML188 provides an excellent starting point for the further design and refinement of 3CLpro inhibitors that act by a noncovalent mechanism of action.
仅供研究使用。 我们不向患者出售。
化学信息
| 名称 | ML188 |
|---|---|
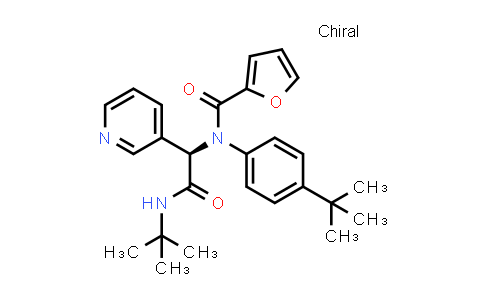
| Iupac 化学名称 | N-[(1R)-2-(tert-Butylamino)-2-oxo-1-pyridin-3-ylethyl]-N-(4-tert-butylphenyl)furan-2-carboxamide |
| 同义词 | ML188; ML-188; ML 188; |
| 英文同义词 | ML188; ML-188; ML 188; |
| 分子式 | C26H31N3O3 |
| 分子量 | 433.552 |
| Smile | O=C(C1=CC=CO1)N([C@H](C2=CC=CN=C2)C(NC(C)(C)C)=O)C3=CC=C(C(C)(C)C)C=C3 |
| InChiKey | JXGIYKRRPGCLFV-JOCHJYFZSA-N |
| InChi | InChI=1S/C26H31N3O3/c1-25(2,3)19-11-13-20(14-12-19)29(24(31)21-10-8-16-32-21)22(18-9-7-15-27-17-18)23(30)28-26(4,5)6/h7-17,22H,1-6H3,(H,28,30)/t22-/m1/s1 |
| Cas号 | 1417700-13-0 |
| 相关CAS号 |
订购信息
| 包装 | 价格 | 库存 | 纯度 | 备货期 |
|---|---|---|---|---|
| 大货 | 询价 | 询价 | 询价 |
| 纯度 | 98% Min. |
|---|---|
| 处理方式 | |
| 海关编码 |
| Targets | |
|---|---|
| Mechanism | |
| Cell study | |
| Animal study | |
| Clinical study |
1: Berry M, Fielding B, Gamieldien J. Human coronavirus OC43 3CL protease and the potential of ML188 as a broad-spectrum lead compound: homology modelling and molecular dynamic studies. BMC Struct Biol. 2015 Apr 28;15:8. doi: 10.1186/s12900-015-0035-3. PMID: 25928480; PMCID: PMC4411765.
2: Turlington M, Chun A, Tomar S, Eggler A, Grum-Tokars V, Jacobs J, Daniels JS, Dawson E, Saldanha A, Chase P, Baez-Santos YM, Lindsley CW, Hodder P, Mesecar AD, Stauffer SR. Discovery of N-(benzo[1,2,3]triazol-1-yl)-N-(benzyl)acetamido)phenyl) carboxamides as severe acute respiratory syndrome coronavirus (SARS-CoV) 3CLpro inhibitors: identification of ML300 and noncovalent nanomolar inhibitors with an induced-fit binding. Bioorg Med Chem Lett. 2013 Nov 15;23(22):6172-7. doi: 10.1016/j.bmcl.2013.08.112. Epub 2013 Sep 7. PMID: 24080461; PMCID: PMC3878165.
3: Turlington M, Chun A, Jacobs J, Dawson E, Daniels JS, Saldanha A, Chase P, Hodder P, Eggler A, Tokars V, Mesecar A, Lindsley CW, Stauffer SR. Non-covalent triazole-based inhibitors of the SARS main proteinase 3CLpro. 2012 Apr 9 [updated 2013 Mar 14]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23762941.
4: Jacobs J, Zhou S, Dawson E, Daniels JS, Hodder P, Tokars V, Mesecar A, Lindsley CW, Stauffer SR. Discovery of non-covalent inhibitors of the SARS main proteinase 3CLpro. 2010 Oct 30 [updated 2013 Feb 28]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23658941.
5: Jacobs J, Grum-Tokars V, Zhou Y, Turlington M, Saldanha SA, Chase P, Eggler A, Dawson ES, Baez-Santos YM, Tomar S, Mielech AM, Baker SC, Lindsley CW, Hodder P, Mesecar A, Stauffer SR. Discovery, synthesis, and structure-based optimization of a series of N-(tert-butyl)-2-(N-arylamido)-2-(pyridin-3-yl) acetamides (ML188) as potent noncovalent small molecule inhibitors of the severe acute respiratory syndrome coronavirus (SARS-CoV) 3CL protease. J Med Chem. 2013 Jan 24;56(2):534-46. doi: 10.1021/jm301580n. Epub 2013 Jan 3. PMID: 23231439; PMCID: PMC3569073.
6: Xue JH, Qian QM, Wang YS, Meng XL, Liu L. Resonance light scattering determination of metallothioneins using levofloxacin-palladium complex as a light scattering probe. Spectrochim Acta A Mol Biomol Spectrosc. 2013 Feb;102:205-11. doi: 10.1016/j.saa.2012.10.016. Epub 2012 Oct 23. PMID: 23220658.
7: Wenning C, Stypmann J, Papavassilis P, Sindermann J, Schober O, Hoffmeier A, Scheld HH, Stegger L, Schäfers M. Left ventricular dilation and functional impairment assessed by gated SPECT are indicators of cardiac allograft vasculopathy in heart transplant recipients. J Heart Lung Transplant. 2012 Jul;31(7):719-28. doi: 10.1016/j.healun.2012.02.018. Epub 2012 Mar 14. PMID: 22425234.